Define Reaction Rates with Mass Action Kinetics
Use mass action kinetics to define zero-order, first-order, second-order, and reversible reactions.
Definition of Mass Action Kinetics
Mass action describes the behavior of reactants and products in an elementary chemical reaction. Mass action kinetics describes this behavior as an equation where the velocity or rate of a chemical reaction is directly proportional to the concentration of the reactants.
Zero-Order Reactions
With a zero-order reaction, the reaction rate does not depend
on the concentration of reactants. Examples of zero-order reactions
are synthesis from a null species, and modeling
a source species that is added to the system at a specified rate.
reaction: null -> P
reaction rate: k mole/second
species: P = 0 mole
parameters: k = 1 mole/secondNote
When specifying a null species, the reaction
rate must be defined in units of amount per unit time not concentration
per unit time.
Entering the reaction above into the software and simulating produces the following result:
Zero-Order Mass Action Kinetics
Note
If the amount of a reactant with zero-order kinetics reaches zero before the end of a simulation, then the amount of reactant can go below zero regardless of the solver or tolerances you set.
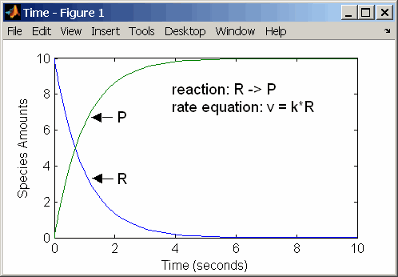
First-Order Reactions
With a first-order reaction, the reaction rate is proportional to the concentration of a single reactant. An example of a first-order reaction is radioactive decay.
reaction: R -> P
reaction rate: k*R mole/(liter*second)
species: R = 10 mole/liter
P = 0 mole/liter
parameters: k = 1 1/second
Entering the reaction above into the software and simulating produces the following results:
First-Order Mass Action Kinetics
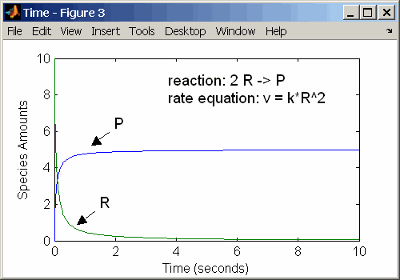
Second-Order Reactions
A second-order reaction has a reaction rate that is proportional
to the square or the concentration of a single reactant or proportional
to two reactants. Notice the space between the reactant coefficient
and the name of the reactant. Without the space, 2R would
be considered the name of a species.
reaction: 2 R -> P
reaction rate: k*R^2 mole/(liter*second)
species: R = 10 mole/liter
P = 0 mole/liter
parameters: k = 1 liter/(mole*second)
Entering the reaction above into the software and simulating produces the following results:
Second-Order Kinetics with Single Reactant
With two reactants, the reaction rate depends on the concentration of two of the reactants.
reaction: R1 + R2 -> P
reaction rate: k*R1*R2 mole/(liter*second)
species: R1 = 10 mole/liter
R2 = 8 mole/liter
P = 0 mole/liter
parameters: k = 1 liter/(mole*second)
Enter the reaction above into the software and simulating produces the following results. There is a difference in the final values because the initial amount of one of the reactants is lower than the other. After the first reactant is used up, the reaction stops.
Second-Order Kinetics with Two Reactants

Reversible Mass Action
You can model reversible reactions with two separate reactions or with one reaction. With a single reversible reaction, the reaction rates for the forward and reverse reactions are combined into one expression. Notice the angle brackets before and after the hyphen to represent a reversible reaction.
reaction: R <-> P
reaction rate: kf*R - kr*P mole/(liter*second)
species: R = 10 mole/liter
P = 0 mole/liter
parameters: kf = 1 1/second
kr = 0.2 1/second
Entering the reaction above into the software and simulating
produces the following results. At equilibrium when the rate of the
forward reaction equals the reverse reaction, v = kf*R -
kr*P = 0 and P/R = kf/kr.
