bioinfo.pipeline.block.SRAFasterqDump
Description
An SRAFasterqDump block enables you to download sequence read
data in the FASTQ or FASTA format from SRA (Sequence Read Archive) [1].
bioinfo.pipeline.block.SRAFasterqDump requires the SRA Toolkit for Bioinformatics Toolbox™. If this support package is not installed, then the function provides a download
link. For details, see Bioinformatics Toolbox Software Support Packages.
Creation
Syntax
Description
b = bioinfo.pipeline.block.SRAFasterqDumpSRAFasterqDump block.
b = bioinfo.pipeline.block.SRAFasterqDump(options)options.
b = bioinfo.pipeline.block.SRAFasterqDump(Name=Value)FastaOutput
name-value argument. The name-value arguments sets the property names and values of an
SRAFasterqDumpOptions object. These property values are assigned to the
Options property of the block.
Input Arguments
SRAFasterqDump options, specified as an SRAFasterqDumpOptions object, string scalar, or character vector.
If you specify a string scalar or character vector, it must be in the
fasterq-dump original syntax (prefixed by a dash).
Data Types: char | string
Name-Value Arguments
Specify optional pairs of arguments as
Name1=Value1,...,NameN=ValueN, where Name is
the argument name and Value is the corresponding value.
Name-value arguments must appear after other arguments, but the order of the
pairs does not matter.
Example: b =
bioinfo.pipeline.block.SRAFasterqDump(FastaOutput=true) specifies to download
the FASTA-formatted file.
Flag to append new data to the output file instead of overwriting it, specified as a numeric
or logical 1 ( true) or 0 (false). By default, the
output file is overwritten with new data.
Data Types: double | logical
Flag to concatenate sequence information pertaining to each spot, specified as a
numeric or logical 1 (true) or 0 (false). By
default, the software does not concatenate the information pertaining to each spot. That
is, the software writes four lines of FASTQ or two lines of FASTA into one output file
for each spot. For details, see FASTQ/FASTA concatenated.
Data Types: double | logical
Additional commands, specified as a character vector or string scalar.
The commands must be in the native syntax (prefixed by one or two dashes). Use this option to apply undocumented flags and flags without corresponding MATLAB® properties.
Example: ExtraCommand="--fasta-ref-tbl --internal-ref"
Data Types: char | string
Flag to save the output in the FASTA format, specified as a numeric or logical
1 (true) or 0 (false). The default
output format is the FASTQ format.
Data Types: double | logical
Flag to split sequence information pertaining to each spot without
preserving the spot order, specified as a numeric or logical 1 (true)
or 0 (false).
If the value is true, the software splits the sequence information
in each spot is into reads. For each read, two lines of FASTA are written into the
single output file. Setting FastaOutputUnsorted=true is the same as
setting SplitType=SplitSpot, with the following exceptions:
With
FastaOutputUnsorted=true, the original order of the spots and reads is not preserved, andFastaOutputUnsortedname-value argument is exclusively for the FASTA output.This setting is faster than the
SplitSpotoption and does not use temporary files.
Data Types: double | logical
String of bases used to filter the output, specified as a string scalar. The output is filtered by comparing it to the specified string of bases and keeping reads that include the specified string of bases.
Data Types: string
Flag to include all object properties with
corresponding default values when converting properties to the original option syntax,
specified as a numeric or logical 1 (true) or 0
(false). You can convert properties to the original syntax
prefixed by one or two dashes (such as '-e 8 --split-file') by using
the getCommand function.
When IncludeAll=false and you call
getCommand(optionsObject), the software converts only the
specified properties. If the value is true,
getCommand converts all available properties, using default
values for unspecified properties, to the original syntax.
Note
If you set IncludeAll to true, the software
converts all available properties, using default values for unspecified properties. The
only exception is when the default value of a property is NaN,
Inf, [], '', or
"". In this case, the software does not translate the
corresponding property.
Data Types: logical | double
Flag to include technical reads in the downloaded files,
specified as a numeric or logical 1
(true) or 0
(false).
Data Types: double | logical
Minimum length required for a read to be included in the output, specified as a nonnegative integer. By default, no read is filtered out.
Data Types: double
Number of parallel threads to use, specified as a positive integer. The software runs threads on separate processors or cores. Increasing the number of threads generally improves the runtime significantly, but also increases the memory footprint.
Data Types: double
Folder where the output files are saved, specified as a character vector or string scalar. By default, the software saves the files in the current directory.
Data Types: char | string
Base name of the output files, specified as a character vector or string scalar. The default base name is the accession run number.
Data Types: char | string
Method used to split sequence information pertaining to each spot, specified as one of the following:
"SplitThree"— The software splits spots into reads. For each read, the software writes four lines of FASTQ or two lines of FASTA. For spots with two reads, the software produces*_1.fastqand*_2.fastqfiles. The software places unmated reads in*.fastq. If the accession does not have any spot with one single read, the software does not create a*.fastqfile. For details, see FASTQ/FASTA split 3."SplitSpot"— The software splits spots into reads. For each read, the software writes four lines of FASTQ or two lines of FASTA. All the reads are saved to a single output file. For details, see FASTQ/FASTA split spot."SplitFiles"— The software splits spots into reads. For each read, the software writes four lines of FASTQ or two lines of FASTA. The software assigns each read a number n, where 1 ≤ n ≤ 5, and then saves each nth read to the nth file (*_n.fastq). For details, see FASTQ/FASTA split file.
By default, the reads refer to biological reads only. However, if you set
IncludeTechnical to true, then the software
also includes the technical reads in the output files.
Data Types: char | string
Properties
Function to handle errors from the run
method of the block, specified as a function handle. The handle specifies the function to call
if the run method encounters an error within a pipeline. For the pipeline to continue after a
block fails, ErrorHandler must return a structure that is compatible with
the output ports of the block. The error handling function is called with the following two inputs:
Structure with these fields:
Field Description identifier Identifier of the error that occurred message Text of the error message index Linear index indicating which block process failed in the parallel run. By default, the index is 1 because there is only one run per block. For details on how block inputs can be split across different dimensions for multiple run calls, see Bioinformatics Pipeline SplitDimension. Input structure passed to the
runmethod when it fails
Data Types: function_handle
This property is read-only.
Input ports of the block, specified as a structure. The field
names of the structure are the names of the block input ports, and the field values are bioinfo.pipeline.Input objects. These objects describe the input port behaviors.
The input port names are the expected field names of the input structure that you pass to the
block run method.
The SRAFasterqDump block Inputs structure has
the following field SRRID, which contains the accession numbers. This
input is required and must be satisfied.
Data Types: struct
This property is read-only.
Output ports of the block, specified as a structure. The field
names of the structure are the names of the block output ports, and the field values are bioinfo.pipeline.Output objects. These objects describe the output port behaviors.
The field names of the output structure returned by the block run method
are the same as the output port names.
The SRAFasterqDump block Outputs structure has
the following fields: Reads, Reads_1,
Reads_2, Reads_3, Reads_4,
Reads_5. The field values are the output filenames. The total
number of output files varies depending on the SplitType option and
the accession run number.
The Reads field corresponds to the single output file produced
when you specify SplitType="SplitSpot". The
Reads_n fields, where 1 ≤ n ≤
5, correspond to the output files produced when you specify
SplitType="SplitThree" or
SplitType="SplitFiles". For details, see SplitType.
Data Types: struct
SRAFasterqDump options, specified as an SRAFasterqDumpOptions object. The default value is a default
SRAFasterqDumpOptions object.
Object Functions
compile | Perform block-specific additional checks and validations |
copy | Copy array of handle objects |
emptyInputs | Create input structure for use with run method |
eval | Evaluate block object |
run | Run block object |
Examples
Import the pipeline and block objects needed for the example so that you can create these objects without specifying the entire namespace.
import bioinfo.pipeline.Pipeline import bioinfo.pipeline.block.*
Create a pipeline.
P = Pipeline;
Create an SRAFasterqDump block and specify the accession number SRR11846824 as the block input. SRR11846824 has two reads per spot and no unaligned reads.
SRAFQDump = SRAFasterqDump;
SRAFQDump.Inputs.SRRID.Value = "SRR11846824";
addBlock(P,SRAFQDump);Run the pipeline to download the corresponding FASTQ files from SRA for the specified accession number.
run(P);
Get the results of the SRAFQDump block.
R = results(P,SRAFQDump)
R = struct with fields:
Reads: [1×1 bioinfo.pipeline.datatype.Incomplete]
Reads_1: [1×1 bioinfo.pipeline.datatype.File]
Reads_2: [1×1 bioinfo.pipeline.datatype.File]
Reads_3: [1×1 bioinfo.pipeline.datatype.Incomplete]
Reads_4: [1×1 bioinfo.pipeline.datatype.Incomplete]
Reads_5: [1×1 bioinfo.pipeline.datatype.Incomplete]
View the names of the downloaded files by using the unwrap function.
unwrap(R.Reads_1) unwrap(R.Reads_2)
By default, the block uses the SplitType="SplitThree" option and downloads only biological reads. Specifically, the block splits spots into reads. For spots with two reads, the block produces *_1.fastq and *_2.fastq and displays them in the Reads_1 and Reads_2 fields, respectively. The block saves any unaligned reads in a *.fastq file and displays it in the Reads field. Because this accession has no unaligned reads, the block did not produce a *.fastq file, and the Reads field is returned as Incomplete. Reads_3, Reads_4, and Reads_5 are also Incomplete because of the usage of SplitType="SplitThree". For more details on the block output behavior, see Outputs.
You can specify other download options using the SRAFasterqDumpOptions. For instance, to download the FASTA-formatted file, specify FastaOutput=true and rerun the block.
opt = SRAFasterqDumpOptions; opt.FastaOutput = true; SRAFQDump.Options = opt;
You can also download SAM files from SRA using the SRASAMDump block.
SRASDump = SRASAMDump;
Specify the accession number to download.
SRASDump.Inputs.SRRID.Value = "SRR11846824";Specify the options using an SRASAMDumpOptions object. For instance, set the output filename and compress the output file using bzip2.
samdumpopt = SRASAMDumpOptions;
samdumpopt.BZip2 = 1;
samdumpopt.OutputFileName = "SRR11846824.sam.bz2"samdumpopt =
SRASAMDumpOptions with properties:
Default properties:
ExtraCommand: ""
FastaOutput: 0
FastqOutput: 0
GZip: 0
HideIdentical: 0
IncludeAll: 0
MinMapQuality: 0
OutputPrimary: 0
OutputUnaligned: 0
Version: "3.0.6"
Modified properties:
BZip2: 1
OutputFileName: "SRR11846824.sam.bz2"
SRASDump.Options = samdumpopt;
Add the block to the pipeline and run the pipeline.
addBlock(P,SRASDump); run(P);
Get the block results.
R2 = results(P,SRASDump);
View the names of the output files by using the unwrap function.
unwrap(R2.OutputFiles)
After downloading the files, you can use them for downstream analyses. For instance, you can run bowtie2 to map the reads to the reference sequence, and then visualize the mapped reads in the Genomics Viewer app.
First, download the C. elegans reference sequence.
celegans_refseq = fastaread("https://s3.amazonaws.com/igv.broadinstitute.org/genomes/seq/ce11/ce11.fa");Save the Chromosome 3 reference data in a FASTA file.
celegans_chr3 = celegans_refseq(3).Sequence;
fastawrite("celegans_chr3.fa",celegans_chr3);Create a FileChooser block to select the Chromosome 3 reference file.
fcRef = FileChooser;
fcRef.Files = fullfile(pwd,"celegans_chr3.fa");
addBlock(P,fcRef);Build a set of index files using the Bowtie2Build block. Set the base name of the index files and the name of the reference FASTA file.
buildIndex = Bowtie2Build; buildIndex.Inputs.IndexBaseName.Value = "celegans_chr3_index"; addBlock(P,buildIndex); connect(P,fcRef,buildIndex,["Files","ReferenceFASTAFiles"]); run(P);
Align reads to the reference using the Bowtie2 block. Create the block and then connect it to buildIndex and SRAFQDump blocks.
alignReads = Bowtie2; alignReads.OutFilename = "SRR11846824_mapped.sam"; addBlock(P,alignReads); connect(P,buildIndex,alignReads,["IndexBaseName","IndexBaseName"]); connect(P,SRAFQDump,alignReads,["Reads_1","Reads1Files";"Reads_2","Reads2Files"]); run(P);
Bowtie2 produces a SAM file. To visualize the mapped reads in the Genomics Viewer app, convert the SAM file to a BAM file.
First, make a UserFunction block to create a BioMap object from the SAM file.
biomapObj = UserFunction; biomapObj.Function = "BioMap"; biomapObj.RequiredArguments = "inputSAM"; biomapObj.OutputArguments = "biomapObject"; addBlock(P,biomapObj);
Next, connect the biomapObj block to the alignReads block, which provides the SAM file needed. Suppress two informational warnings issued during the creation of a BioMap object.
connect(P,alignReads,biomapObj,["SAMFile","inputSAM"]); w = warning; warning("off","bioinfo:BioMap:BioMap:UnsortedReadsInSAMFile"); warning("off","bioinfo:saminfo:InvalidTagField"); run(P); warning(w); % Restore warnings
Use the write method of the BioMap object to convert the SAM file to a BAM file.
sam2bam = UserFunction; sam2bam.Function = "write"; sam2bam.RequiredArguments = ["biomapObj","BAMFileName"]; sam2bam.NameValueArguments = "Format"; sam2bam.Inputs.BAMFileName.Value = "../../../SRR11846824_mapped.bam"; sam2bam.Inputs.Format.Value = "BAM"; addBlock(P,sam2bam); connect(P,biomapObj,sam2bam,["biomapObject","biomapObj"]); run(P);
Create a FileChooser block to select the generated BAM file.
fcBAM = FileChooser;
fcBAM.Files = fullfile(pwd,"SRR11846824_mapped.bam");
addBlock(P,fcBAM);Create a FileChooser block to select the C. elegans cytoband file, which is provided with the toolbox.
fcCyto = FileChooser;
fcCyto.Files = fullfile(pwd,"celegans_cytoBandIdeo.txt.gz");
addBlock(P,fcCyto);View the alignment data using the Genomics Viewer app.
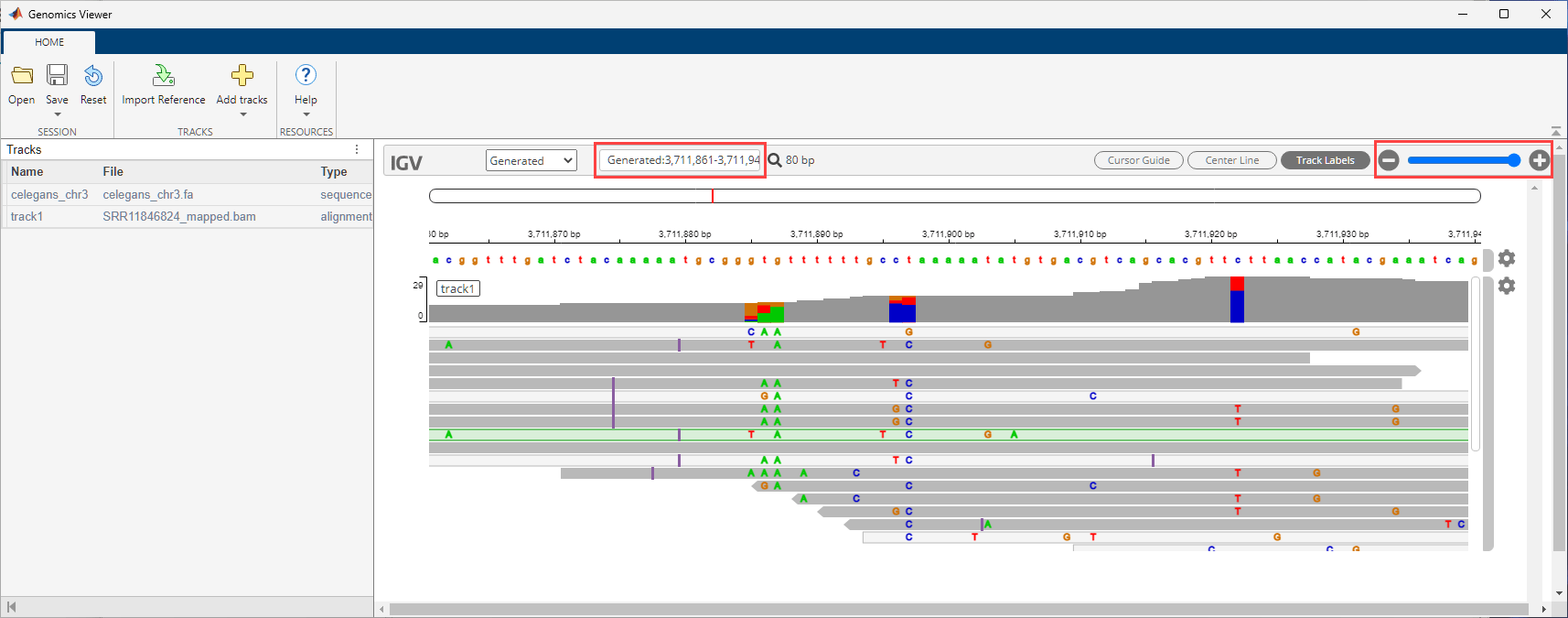
gv = GenomicsViewer; addBlock(P,gv); connect(P,fcRef,gv,["Files","Reference"]); connect(P,fcCyto,gv,["Files","Cytoband"]); connect(P,fcBAM,gv,["Files","Tracks"]); run(P);
Use the zoom slider to zoom in and see the features. Or you can enter the following in the search text box: Generated:3,711,861-3,711,940.
Delete the pipeline results and downloaded files.
deleteResults(P,IncludeFiles=true);
References
[1] SRA Toolkit Development Team https://github.com/ncbi/sra-tools/wiki/01.-Downloading-SRA-Toolkit
Version History
Introduced in R2024a
MATLAB Command
You clicked a link that corresponds to this MATLAB command:
Run the command by entering it in the MATLAB Command Window. Web browsers do not support MATLAB commands.
选择网站
选择网站以获取翻译的可用内容,以及查看当地活动和优惠。根据您的位置,我们建议您选择:。
您也可以从以下列表中选择网站:
如何获得最佳网站性能
选择中国网站(中文或英文)以获得最佳网站性能。其他 MathWorks 国家/地区网站并未针对您所在位置的访问进行优化。
美洲
- América Latina (Español)
- Canada (English)
- United States (English)
欧洲
- Belgium (English)
- Denmark (English)
- Deutschland (Deutsch)
- España (Español)
- Finland (English)
- France (Français)
- Ireland (English)
- Italia (Italiano)
- Luxembourg (English)
- Netherlands (English)
- Norway (English)
- Österreich (Deutsch)
- Portugal (English)
- Sweden (English)
- Switzerland
- United Kingdom (English)