Khawaja Medical Technology Achieves IEC 62304 Compliance for ECG Analysis Software
“We’ve seen a significant return on the investment we made in Model-Based Design, including higher quality, reduced development times, and faster ISO and IEC certification.”
Challenge
Automate ECG signals analysis to detect cardiac abnormalities
Solution
Use Model-Based Design and the IEC Certification Kit reference workflow to model, simulate, and generate production code for ECG analysis software
Results
- Development time reduced by 40%
- ISO 13485 and IEC 62304 certification accelerated
- Prototype built in months, not years
Electrocardiogram (ECG) data analysis is essential for the recognition and treatment of cardiac diseases. It is applied in a variety of diagnostic settings, including preclinical, clinical, ambulatory, and in-home settings, as well as in clinical trials of new drugs.
As part of its cardiac drug development and approval process, a pharmaceutical company must investigate the new drug’s effect on the heart. This involves analyzing ECG signals to identify abnormalities and ensure cardiac drug safety.
Engineers at Khawaja Medical Technology have developed novel and advanced algorithms that fully automate ECG signal analysis. The algorithms enable real-time monitoring and analysis of ECG signals from a subject who is resting, exercising, or wearing a Holter monitor (used to track heart rhythms over a period of days). Khawaja Medical Technology accelerated development of the software by using Model-Based Design with MATLAB® and Simulink®.
“We’ve been using Model-Based Design from the first day of our company,” says Dr. Antoun Khawaja, CEO and chief scientific officer at Khawaja Medical Technology. “By enabling us to rapidly create prototypes and ensure compliance with ISO standards for medical devices, Model-Based Design reduced development time for high-quality ECG analysis software by 40%.”
Challenge
The Khawaja Medical Technology engineering team needed to develop sophisticated ECG signal processing and analysis algorithms; they also needed to ensure compliance with numerous international standards governing medical device software, including IEC 62304. Further, the team needed to support a range of cardiac analysis use cases and a variety of deployment options, including web-based algorithm services and algorithms delivered as licensed software or as embedded software for certified medical devices.
Solution
Khawaja Medical Technology engineers used Model-Based Design with MATLAB and Simulink as part of an IEC 62304–compliant software development process to develop and deploy algorithms for automated ECG analysis.
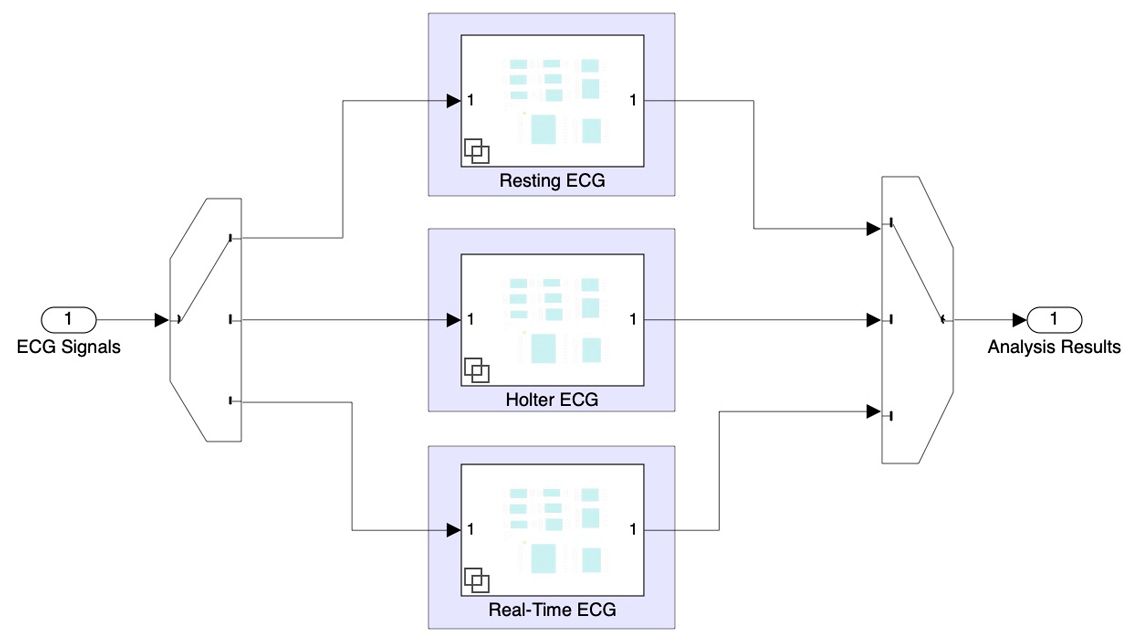
The team defined a multitiered software architecture with a user interface layer, a data flow layer, and a low-level signal processing and classification layer.
They developed a set of MATLAB classes, which they used to create reusable System objects™ for the signal processing and classification layer. These System objects perform a variety of tasks, such as detecting peaks in ECG signals, measuring signal characteristics, classifying arrhythmias, and diagnosing ventricular hypertrophy, myocardial infarction, and other heart conditions.
Working in Simulink and Stateflow®, the team used state machines to model the application logic for the data flow layer, which coordinates the exchange of signals and data with the System objects.
For the user interface layer, the team developed models for specific ECG applications, such as resting ECG analysis, real-time monitoring, and Holter ECG analysis. Requirements for these applications were managed in Requirements Toolbox™ and traced to the equivalent Simulink model elements and generated code.
Using Simulink Check™, the team checked their models for compliance with modeling guidelines and standards, including IEC 62304. They authored and executed simulation-based tests with Simulink Test™, traced the tests to requirements, and measured test coverage with Simulink Coverage™.
Next, they generated C code from the models for both software-only and hardware products. They verified the generated code via software-in-the-loop testing, reusing the test cases that they had applied in simulation-based tests.
The Khawaja Medical Technology is certified according to ISO 13485 by TÜV SÜD. Because it is ISO 13485 compliant, Khawaja Medical Technology conforms to a number of international standards, including IEC 62304. Khawaja Medical Technology fulfills all software life-cycle requirements of the IEC 62304 standard by employing a reference workflow for Model-Based Design provided by MathWorks.
The first medical device using the company’s algorithms is pending certification and is expected to be available in 2021.
Results
- Development time reduced by 40%. “With Model-Based Design, we reduced development time and costs by about 40% compared with traditional approaches,” says Dr. Khawaja. “We saved a great deal of time by following the reference workflow from IEC Certification Kit to verify and validate our models and generated code.”
- ISO 13485 and IEC 62304 certification accelerated. “Our certification and audit with TÜV SÜD for ISO 13485, including IEC 62304, went smoothly because we used Model-Based Design from the start,” says Dr. Khawaja. “We used code generation and verification tools—as well as a reference workflow—that were all pre-certified for the standard, so we simply had to demonstrate that we were following the workflow.
- Prototype built in months, not years. “Rapid prototyping with MATLAB and Simulink enabled us to demonstrate a working system to our customer in a few months,” notes Dr. Khawaja. “Once we had a working design, we just connected to the target platform and ran the algorithms. With conventional development, it would have taken up to two years to see the first results.”